Neuroscience Updates vol. 115
December 16th 2024
Check out latest research updates in the field
• Long-term memory may not depend on short-term memory after all
• The first complete neural map of the circadian clock was unveiled
• Cryo-electron tomography unveils synaptic vesicle mysteries
• Learning a new language? sleep may be the key
• Spider brain research leads to Alzheimer’s discovery
Neuroscience market
The global neuroscience market size was valued at USD 28.4 billion in 2016 and it is expected to reach USD 38.9 billion by 2027.
The latest news and research
Formation of long-term memory without short-term memory revealed by CaMKII inhibition
by Shin ME, Parra-Bueno P, Yasuda R. in Nature Neuroscience.
Researchers from Max Planck Florida Institute for Neuroscience have discovered a new pathway to forming long-term memories in the brain. Their work suggests that long-term memory can form independently of short-term memory, a finding that opens exciting possibilities for understanding memory-related conditions.
Our brain works diligently to record our experiences into memories, creating representations of our daily events that stay with us for short time periods. Current scientific theories of memory formation suggest that short-term memories are stored in what we can imagine as a temporary art exhibition in our brain before being cleared out for representations of new experiences. A tiny fraction of these short-term memories — those most relevant to us — are moved to a more permanent exhibit, our long-term memory, where they are stored for days, years, or decades.
The most prevalent theories suggest this is a linear process. Our experience is encoded into a short-term memory, which is then consolidated into a long-term memory. However, a new study by Dr. Myung Eun Shin, Dr. Paula Parra-Beuno, and MPFI Scientific Director Dr. Ryohei Yasuda suggests that there may be another way to long-term memory formation.
“This discovery is akin to finding a secret pathway to a permanent gallery in the brain,” said Dr. Shin, the study’s lead author. “The prevailing theory suggested a single pathway, where short-term memories were consolidated into long-term memories. However, we now have strong evidence of at least two distinct pathways to memory formation — one dedicated to short-term memories and another to long-term memories. This could mean our brains are more resilient than previously thought.”
The research team focused on a specific enzyme in neurons called CaMKII, which is critical for short-term memory formation. Previously, they developed an optogenetic approach that uses light to temporarily deactivate CaMKII. With this tool in hand, the team set out to use light to block short-term memory formation in a mouse.
Mice prefer dark spaces and, when given a choice, will immediately enter a dark space from a brightly lit one. However, if a mouse is frightened in a particular dark space, the memory of the frightening experience will alter its behavior, and the mouse will avoid entering the dark space again. When the research team used their tool to disrupt memory formation, even those mice that had a frightening experience an hour earlier entered the dark space, suggesting they had no memory of the experience. The scientists had successfully blocked short-term memory formation.
What happened next was surprising to the research team. A day, week, or even a month later, these mice were altering their behavior to avoid where they were previously frightened. Mice that didn’t seem to remember the frightening experience an hour after it occurred, showed clear evidence of remembering at later times. In other words, blocking short-term memory of the event did not disrupt long-term memory.
“We were initially quite surprised by this observation, as it was inconsistent with how we thought memories were formed. We didn’t think it was possible to have a long-term memory of an event without a short-term memory. However, when we repeated these experiments and used multiple tools and approaches to verify our findings, we were convinced,” describes Dr. Shin. “Rather than long-term memory formation being a linear process, that requires short-term memory, a parallel pathway to long-term memory formation that bypasses short-term memory must exist.”
This study has changed the model of how memories are formed in the brain. Significant scientific advances often come after previous models of understanding are overturned, and the team is excited to see where this line of research will take them.
“This new finding has revised our understanding. We are now investigating how this newly discovered pathway to long-term memory formation occurs. We are excited to see what we can learn and what this could mean for preserving long-term memory retention, even when short-term memory is compromised by aging or cognitive impairment,” says Dr. Yasuda.
Synaptic connectome of the Drosophila circadian clock
by Reinhard N, Fukuda A, Manoli G, et al. in Nature Communications
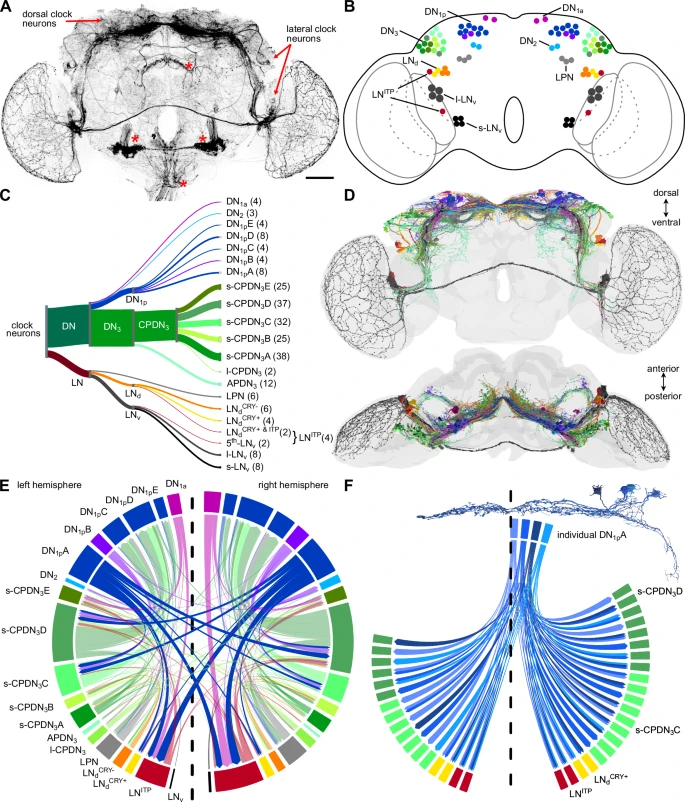
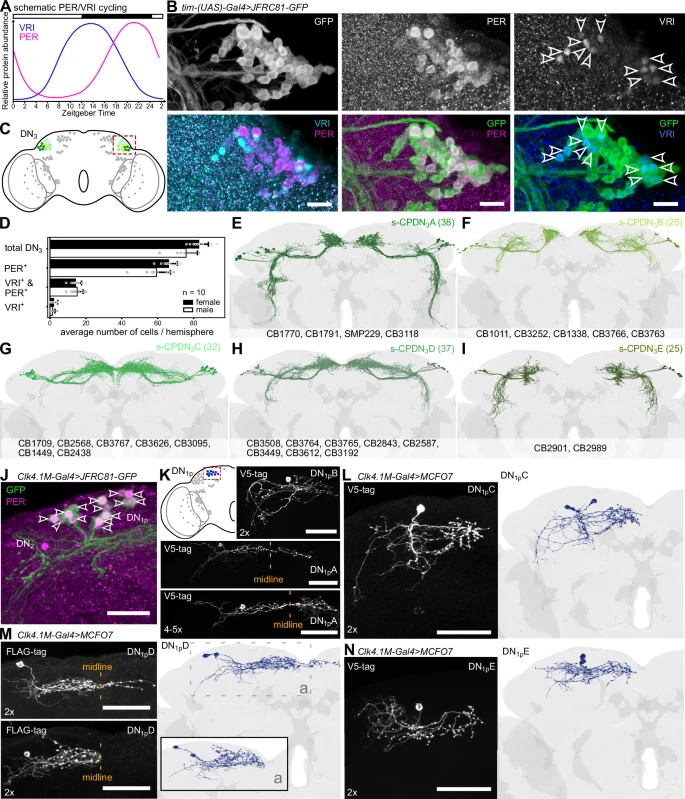
A team of scientists has created the first complete map of the circadian clock in the fruit fly's brain (Drosophila melanogaster), uncovering mechanisms that regulate daily biological rhythms. This study, published in Nature Communications, was led by researchers from the University of Würzburg, Germany, with contributions from the University of Nevada, Reno and Okayama University, Japan.
An internal biological system that regulates physiological and behavioral processes over a 24-hour cycle. It synchronizes functions like sleep, metabolism and reproduction with environmental cues such as light and temperature.
The circadian clock governs a 24-hour cycle of biological processes, including sleep, metabolism and reproduction. In vertebrates, this “master clock” resides in the suprachiasmatic nucleus (SCN) of the brain, which synchronizes rhythms across various tissues. Disruptions in these rhythms are linked to disorders such as insomnia and metabolic conditions. However, due to the complexity of the SCN’s approximately 20,000 neurons, studying its mechanisms can be challenging.
A region of the brain in vertebrates that serves as the central circadian pacemaker. It coordinates the body’s rhythms by signaling peripheral clocks in other tissues.
Clock neurons are specialized neurons involved in the generation and regulation of circadian rhythms. They synchronize with environmental signals to maintain the body’s internal clock.
The fruit fly has emerged as a model organism for studying the circadian clock due to its simpler brain structure, which contains about 140,000 neurons. A recently published connectome of the Drosophila brain — a comprehensive map of all neuronal connections — has provided a unique opportunity to identify and analyze its circadian clock network.
A detailed map of the neural connections within the brain. It enables researchers to study how neurons communicate and organize complex behaviors and processes.
Using the connectome, researchers identified at least 240 neurons involved in the fly’s circadian clock, significantly more than the previously estimated 150. Some of these newly identified neurons share characteristics with clock neurons in vertebrates, suggesting a greater similarity in circadian mechanisms between insects and vertebrates than previously recognized. The researchers also identified specific neuron types that coordinate time-keeping and communication within the clock network.
The circadian clock interacts with brain regions responsible for behaviors like feeding, navigation and hormone regulation. This detailed map allows researchers to trace pathways that link the clock to these processes, providing insights into how rhythmic behaviors are coordinated. Additionally, the study lays the groundwork for investigating circadian dysregulation, which is implicated in conditions such as sleep disorders and metabolic diseases.
The findings expand our understanding of the brain’s role in maintaining daily rhythms and offer a foundation for developing therapeutic approaches to address circadian-related health problems.
Sleep is critical for all sorts of reasons, but a team of international scientists has discovered a new incentive for getting eight hours of sleep every night: it helps the brain to store and learn a new language.
Slow oscillation-spindle coupling predicts sequence-based language learning
by Cross ZR, Helfrich RF, Corcoran AW, et al. J Neuroscience
A study led by the University of South Australia (UniSA) and published in the Journal of Neuroscience has revealed that the coordination of two electrical events in the sleeping brain significantly improves our ability to remember new words and complex grammatical rules
In an experiment with 35 native English-speaking adults, researchers tracked the brain activity of participants learning a miniature language called Mini Pinyin that is based on Mandarin but with similar grammatical rules to English.
Half of the participants learned Mini Pinyin in the morning and then returned in the evening to have their memory tested. The other half learned Mini Pinyin in the evening and then slept in the laboratory overnight while their brain activity was recorded. Researchers tested their progress in the morning.
Lead researcher Dr Zachariah Cross, who did his PhD at UniSA but is now based at Northwestern University in Chicago, says sleep-based improvements were linked to the coupling of slow oscillations and sleep spindles — brainwave patterns that synchronise during NREM sleep.
“This coupling likely reflects the transfer of learned information from the hippocampus to the cortex, enhancing long-term memory storage,” Dr Cross says.
“Post-sleep neural activity showed unique patterns of theta oscillations associated with cognitive control and memory consolidation, suggesting a strong link between sleep-induced brainwave co-ordination and learning outcomes.”
UniSA researcher Dr Scott Coussens says the study underscores the importance of sleep in learning complex linguistic rules.
“By demonstrating how specific neural processes during sleep support memory consolidation, we provide a new perspective on how sleep disruption impacts language learning,” Dr Coussens says. “Sleep is not just restful; it’s an active, transformative state for the brain.”
The findings could also potentially inform treatments for individuals with language-related impairments, including autism spectrum disorder (ASD) and aphasia, who experience greater sleep disturbances than other adults.
Research on both animals and humans shows that slow oscillations improve neural plasticity — the brain’s ability to change and adapt in response to experiences and injury.
“From this perspective, slow oscillations could be increased via methods such as transcranial magnetic stimulation to accelerate aphasia-based speech and language therapy,” Dr Cross says.
In future, the researchers plan to explore how sleep and wake dynamics influence the learning of other complex cognitive tasks.
“Understanding how the brain works during sleep has implications beyond language learning. It could revolutionize how we approach education, rehabilitation, and cognitive training.”
Myelinated glial cells: their proposed role in waste clearance and neurodegeneration in arachnid and human brain
by Fabian-Fine R, Weaver AL, Roman AG, Winters MJ, DeWitt JC. J Compar Neurol.
Researchers from Saint Michael’s College and the University of Vermont have made a groundbreaking new discovery that provides a better understanding of how Alzheimer’s disease develops in the human brain.
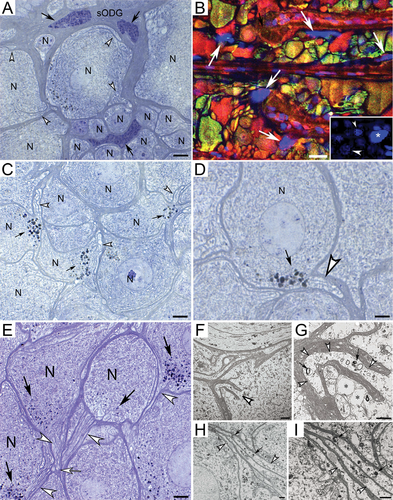
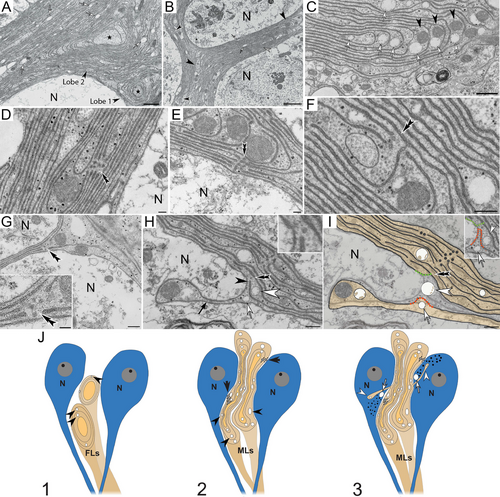
Guided by previous research on spider brains, the scientists uncovered evidence of a “waste canal system” in the human brain that internalizes waste from healthy neurons. They discovered that this system can undergo catastrophic swelling, which leads to the degeneration of brain tissue, a hallmark of Alzheimer’s disease.
With over 50 million affected people worldwide, Alzheimer’s disease is among the leading causes of death in the U.S.
The findings, which have been published by The Journal of Comparative Neurology, offer a compelling new explanation for commonly described brain pathologies observed in Alzheimer’s disease, including amyloid-beta plaques, tau tangles, and spongiform abnormalities.
Supported by the Vermont Biomedical Research Network (VBRN), the research was carried out in collaboration among Dr. Ruth Fabian-Fine (Saint Michael’s College, UVM Robert Larner, M.D. College of Medicine), Dr. John DeWitt (UVM Robert Larner, M.D. College of Medicine, UVM Medical Center), Dr. Adam Weaver (Saint Michael’s College), and Saint Michael’s undergraduate research students Abigail Roman and Melanie Winters, both members of the Class of 2025.
“The Vermont Biomedical Network has been thrilled to support Dr. Fabian-Fine’s research from its initial focus on animal neuroscience to the more recent and potentially groundbreaking emphasis on the cellular basis of human neurodegeneration,” said UVM’s Dr. Christopher Francklyn, the Director of VBRN. “Her exciting work, and the outstanding training she has provided to her undergraduate co-investigators, epitomizes what NIH hopes to accomplish with its national IDEA program.”
Neuroscientist Dr. Fabian-Fine and her team initially investigated the underlying causes for neurodegeneration in Central American wandering spiders that suffer from conditions similar to degenerative diseases in humans. Because the spider neurons were a larger size, the scientists were better able to observe their brain functions. They quickly discovered a waste-internalizing glial canal system that undergoes structural abnormalities in degenerating spider brains, which leads to uncontrolled depletion and death of brain cells.
This discovery prompted Fabian-Fine, a Vermont Center for Cardiovascular and Brain Health Pipeline Investigator, to explore whether a similar system could be found in both rodent and human brain tissue, so she teamed up with neuropathologist Dr. DeWitt at UVM’s Larner College of Medicine. The collaborative undertaking led the scientists to gather overwhelming evidence that neurodegeneration in human and rodent brains may have similar underlying causes compared to those observed in spider brains. The scientists’ report outlines possible underlying causes for neurodegeneration that may offer a promising new avenue for drug development that can address the structural abnormalities that lead to neurodegeneration.
Molecular architecture of synaptic vesicles
by Kravčenko U, Ruwolt M, Kroll J, et al. in PNAS
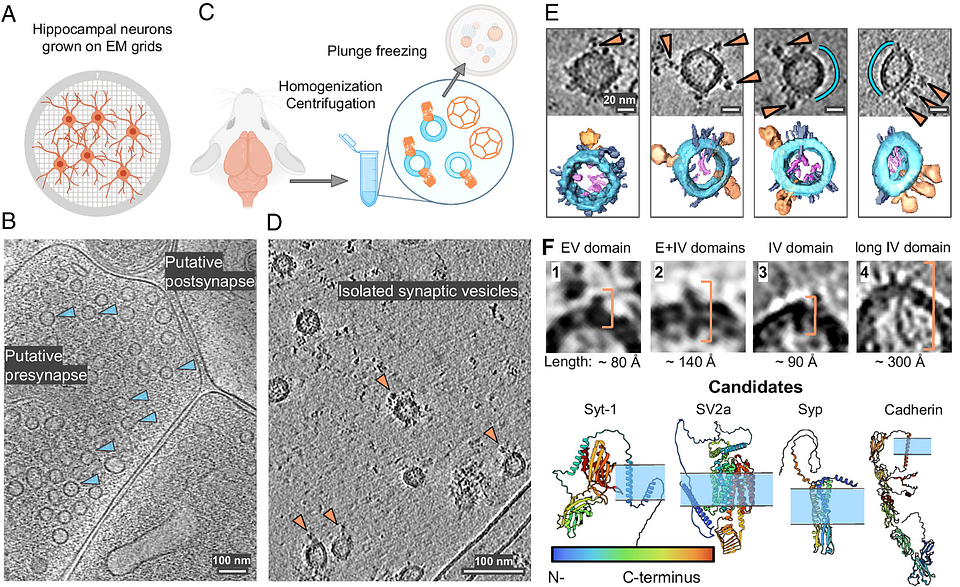
Researchers at the Max Delbrück Center have used cryo-electron tomography to uncover new details of the molecular structure of synaptic vesicles, which help transport neurotransmitters in the brain. The study, published in “PNAS,” could inform therapeutic strategies for psychiatric disorders.
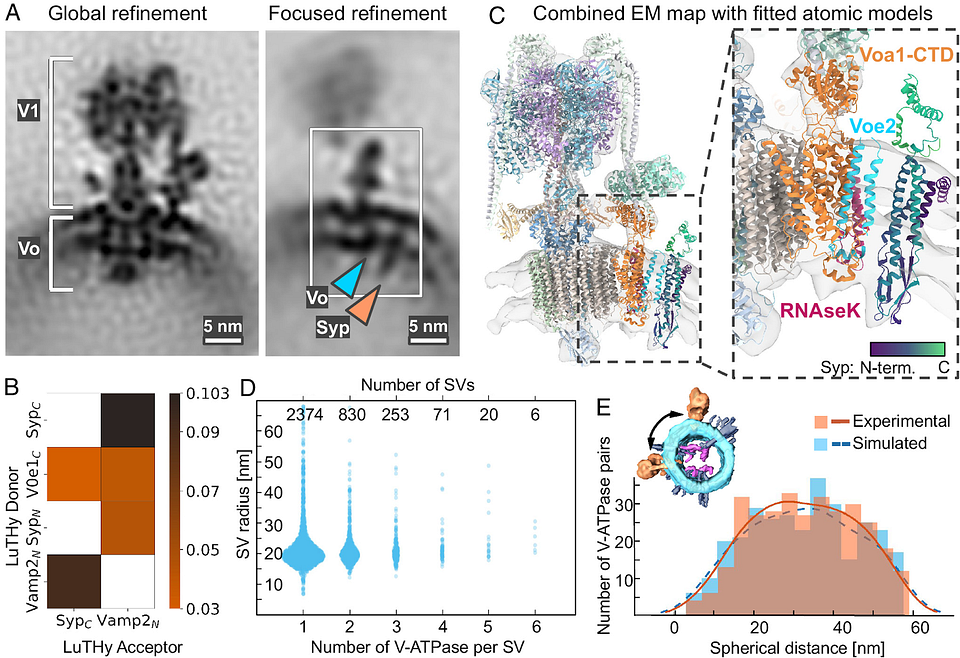
Researchers led by Uljana Kravčenko and her colleagues in the lab of Professor Misha Kudryashev, Group Leader of the In Situ Structural Biology lab at the Max Delbrück Center, have revealed new features of the molecular architecture of synaptic vesicles. Using cryo-electron tomography, the team was able to visualize SVs in 3D and confirm a potentially important protein-protein interaction. They also broadened our understanding of SV function and of how the vesicles are recycled.
“Synaptic vesicles are of paramount importance in brain function and they have been studied for decades. However, previous reports have described the molecular composition of an ‘average’ synaptic vesicle. Our study was able to image individual vesicles at molecular resolution,” says Kudryashev, corresponding author of the paper.
Synaptic versicles — sphere-like structures that store and release neurotransmitters such as dopamine and serotonin, are found in the presynaptic terminals of neurons. They play a crucial role in helping to transmit signals between neurons. The largest protein on their surface is a flower-shaped molecule called V-ATPase. Sitting next to it, is another small protein called synaptophysin. Researchers have thought that the two proteins interact, but no one had directly imaged them until this year, says Kravčenko.
“This study represents one of the first direct visualizations showing the localization of these two proteins on synaptic vesicle membranes,” she explains.
The authors also imaged the locations of partially assembled and empty clathrin cages– lattice-like structures that play a pivotal role in recycling SVs back into cells — inside neurons. These cages were found closer to the cell membrane than had been previously shown, which could be a sign of an energy-efficient mechanism via which SVs are recycled, says Kravčenko, “but we need additional experiments to prove it.”
There are very few images of empty clathrin cages in the literature, she adds. “We show for the first time that empty cages are located closer to the cell membrane. This observation hints at a functional role for the empty cages found in neurons.”
Cryo-electron tomography is an imaging technique that takes 2D images of cryogenically frozen samples at multiple tilt angles to reconstruct three-dimensional volumes of biological samples. It is most often used to study how macromolecules, cellular organelles, or cells are spatially organized, providing structural and contextual insights at sub-nanometer resolution.
Unlike other methods to analyze protein structure such as mass spectrometry or cryo-electron microscopy, cryo-electron tomography enables researchers to observe proteins in their native environment. Other techniques that require many more steps to process samples often lose important structural information.
“Our method preserves the vesicles in their native state, enabling us to image a broad variety of proteins on their surface,” says Kravčenko.
Cryo-electron tomography enabled the researchers to show the spatial organization of proteins and unveiled a persistent association between V-ATPase and synaptophysin, suggesting that the interaction has an important function.
The identified interactions between V-ATPase and synaptophysin offer insights into the molecular mechanisms underlying some neurological disorders. Specifically, those that involve dysfunction in synaptic vesicle recycling and neurotransmission, says Kravčenko.
“Now that we know that these two proteins interact, the information can be used for diagnostics, or to develop treatments for diseases associated with abnormal neurodevelopment.”
Subscribe to Paradigm!
Medium, Twitter, Telegram, Telegram Chat, LinkedIn, and Reddit.
Main sources
Research articles